
Xpert® Carba-R
Detection and differentiation of KPC, NDM, VIM, IMP, and OXA-48 in 50 minutes
Sign in or create a MyCepheid account to add items to cart
Test pack size(s)

10 Tests
GXCARBAR-10
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Collection devices
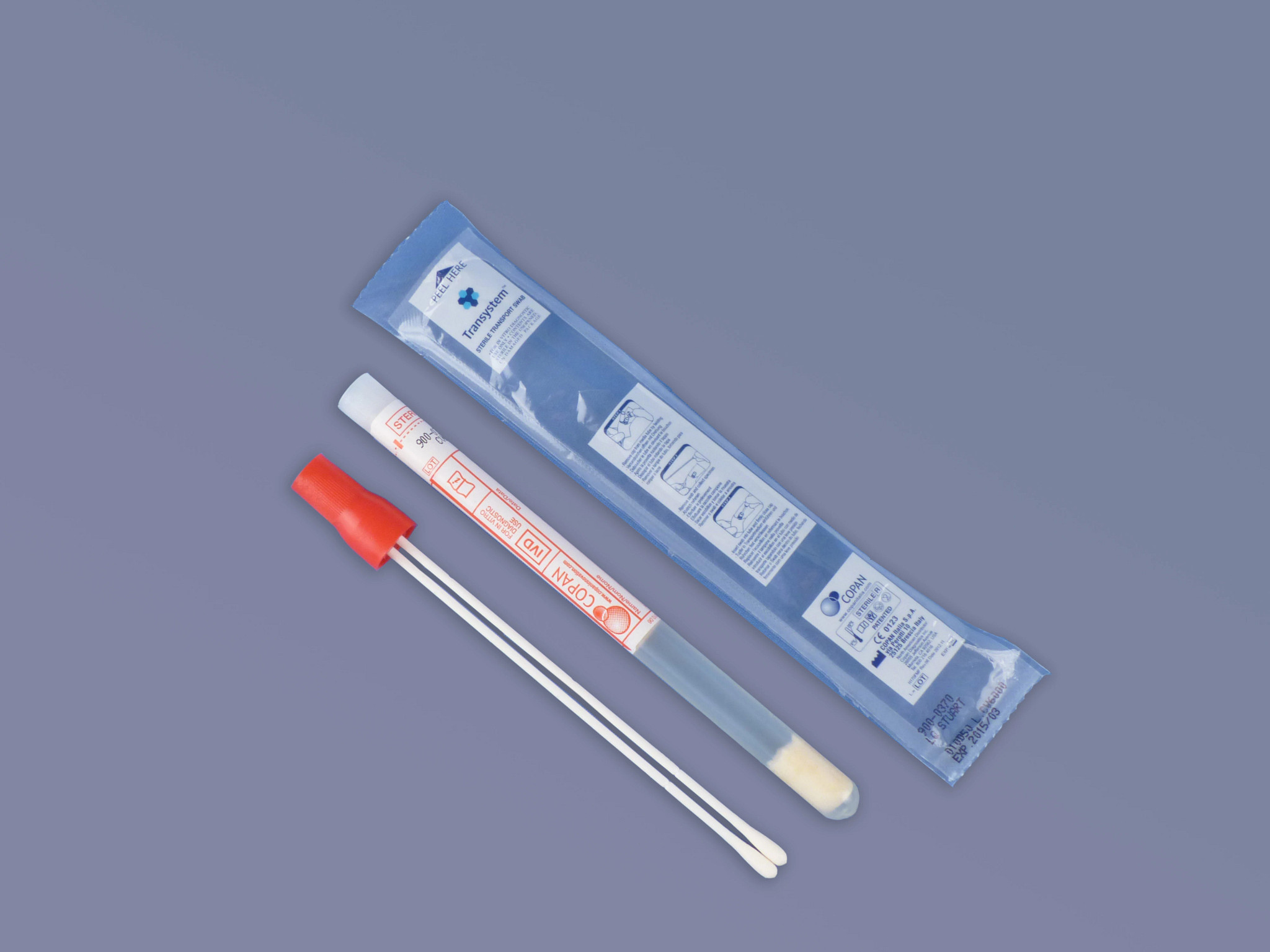
Collection Device (Pack of 50)
900-0370
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Total
{{currency}}
0
Error adding items to cart. If this error persists, please contact Digital Support
The Need
- The emergence and global spread of carbapenemase producing Enterobacterales (CPE) is of great concern to health services worldwide1
- Carbapenem resistance results in increased mortality in hospitalized patients and is associated with higher total hospital costs2
- CPE are multi-drug resistant organisms that can cause serious infections and require interventions in healthcare settings to prevent spread3
- ECDC and CDC recommend comprehensive infection control measures for patients who are colonized or infected with carbapenemase-producing organisms3,4
- 1 Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. Carbapenemase-Producing Organisms: A Global Scourge. Clin Infect Dis. 2018 Apr 3;66(8):1290-1297. doi: 10.1093/cid/cix893. PMID: 29165604; PMCID: PMC5884739.
2 Judd WR, et al. Clinical and economic impact of meropenem resistance in Pseudomonas aeruginosa–infected patients. American Journal of Infection Control. June 2016. http://www.sciencedirect.com/science/article/pii/S0196655316303431
3 CDC Healthcare Facilities: Information about CRE https://www.cdc.gov/hai/organisms/cre/cre-facilities.html
4 ECDC RAPID RISK ASSESSMENT. Carbapenem-resistant Enterobacteriaceae–second update. 26 Sept. 2019. Accessed June 2020 https://www.ecdc.europa.eu/sites/portal/files/documents/carbapenem-resistant-enterobacteriaceae-risk-assessment-rev-2.pdf
The Solution
- Detection and differentiation of patients with the Xpert Carba-R test can quickly alert clinicians and infection preventionists to the presence of gene sequences associated with carbapenem non-susceptibility
- Rapid detection and differentiation of the KPC, NDM, VIM, IMP, and OXA-48 gene sequences from pure colonies helps clinicians optimize patient management and direct therapeutic strategy
- Rectal and perirectal swabs assist with infection control measures
- On-demand identification of the most prevalent carbapenemase gene families enables healthcare systems to prevent onward transmission throughout the patient pathway and facilitates more efficient bed management for improved hospital flow5
The Impact
- Reduced turnaround time enables rapid infection control measure to reduce transmission6,7
- Significant reduction the number of bed-days lost due to CPE compared to culture5
- Significant decrease in CPE colonization and infection rates with fast PCR results8
5 Corless C. et al. Impact of different carbapenemase-producing Enterobacterales screening strategies in a hospital setting. IPIP. 2020 May;3(2):100011.
6 Jin S, Lee JY, Park JY, Jeon MJ. Xpert Carba-R assay for detection of carbapenemase-producing organisms in patients admitted to emergency rooms. Medicine (Baltimore). 2020 Dec 11;99(50):e23410.
7 Ambretti S et al. Total integration of cultural and molecular testing for CPE screening with liquid based microbiology (LBM). Poster presented at ECCMID. 2018 21-24 April. Madrid, Spain.
8 Zhou M, Kudinha T, Du B, Peng J, Ma X, Yang Y, Zhang G, Zhang J, Yang Q, Xu YC. Active Surveillance of Carbapenemase-Producing Organisms (CPO) Colonization With Xpert Carba-R Assay Plus Positive Patient Isolation Proves to Be Effective in CPO Containment. Front Cell Infect Microbiol. 2019 May 14;9:162. doi: 10.3389/fcimb.2019.00162. PMID: 31157176; PMCID: PMC6528581.
6 Jin S, Lee JY, Park JY, Jeon MJ. Xpert Carba-R assay for detection of carbapenemase-producing organisms in patients admitted to emergency rooms. Medicine (Baltimore). 2020 Dec 11;99(50):e23410.
7 Ambretti S et al. Total integration of cultural and molecular testing for CPE screening with liquid based microbiology (LBM). Poster presented at ECCMID. 2018 21-24 April. Madrid, Spain.
8 Zhou M, Kudinha T, Du B, Peng J, Ma X, Yang Y, Zhang G, Zhang J, Yang Q, Xu YC. Active Surveillance of Carbapenemase-Producing Organisms (CPO) Colonization With Xpert Carba-R Assay Plus Positive Patient Isolation Proves to Be Effective in CPO Containment. Front Cell Infect Microbiol. 2019 May 14;9:162. doi: 10.3389/fcimb.2019.00162. PMID: 31157176; PMCID: PMC6528581.










