Fast, Simple, and Accurate PCR Testing for Pediatric Care
Cepheid, a leader in molecular diagnostics, offers solutions that increase pediatric care efficiency while achieving better clinical care.
GeneXpert® Family of Systems Deliver Unmatched Results from Decentralized Testing to the Point of Care
Pediatric care providers play a critical role in the healthcare ecosystem. From managing day-to-day health needs to treating their patients when sick, this range of care needs presents unique challenges. Diagnosing and treating pediatric patients quickly and accurately is the highest priority. Cepheid offers proven purpose-built solutions that increase your practice efficiency while achieving higher patient satisfaction and better clinical care.
Explore the GeneXpert® Xpress system and our extensive respiratory test menu take your Pediatric practice to the next level.

Seamless In-office Testing
|
|
|
|
|
|
Testing as simple as 1, 2, 3
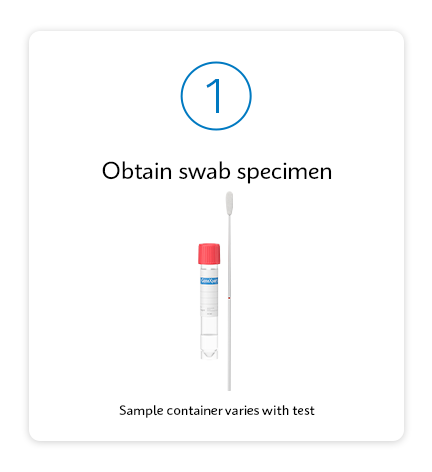
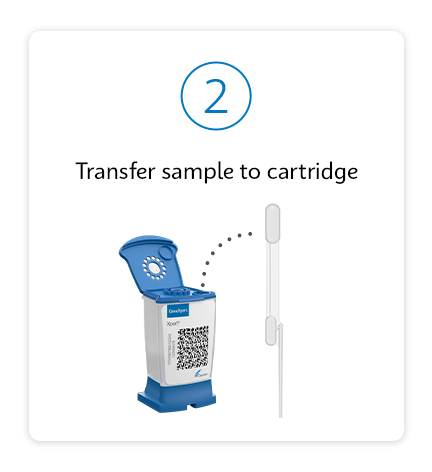

Respiratory Solutions
Build your Pediatric Practice with a Single Solution for Respiratory Care
Test with the confidence of PCR using Cepheid’s GeneXpert® System and Xpress® tests. Standardized CLIA waived molecular testing for any pediatric care setting — from health systems to standalone physician offices and specialty clinics. Achieve accurate, actionable results when and where they are needed the most.

Xpert® Xpress CoV-2/Flu/RSV plus

Xpert® Xpress CoV-2 plus

Xpert® Xpress Flu

Xpert® Xpress Strep A

How Treating Pediatric Patients is Different
Dr. Dov Shapiro talks about the Challenges of treating pediatric patients

How Treating Pediatric Patients is Different
Dr. Dov Shapiro talks about the Challenges of treating pediatric patients
TRANSCRIPT: How Treating Pediatric Patients Is Different
Treating pediatric patients comes with its own challenges. Number one, we're worried about missed school days. Kids need to be in school. When they're not in school, they don't learn, they don't socialize. It's not good for their mental health. The other consideration, when children are out of school, their parents can't go to work. They have to stay home to take care for their kids. We talk over and over again about adult hospitalizations during the COVID-19 pandemic, but the last couple of months with the triple-demic, we've seen pediatric hospitals overwhelmed without beds, ERs holding patients overnight because there's no beds to put them in. Children get sick too, and the ability to accurately diagnose their illness early on and initiate appropriate treatment results in fewer ER visits and less pediatric hospitalizations. It's important to remember that being able to treat kids when they need it, as early as possible, really results in better outcomes.
Educational Materials
Respiratory Testing Brochure (Point of Care)
Co-testing for SARS-CoV-2, flu, and RSV will be critical to detect and differentiate between disease states, enabling accurate patient diagnosis and treatment
White Paper: Leveraging Point-of-Care PCR Testing in Pediatrics
Equipping laboratories, physicians’ offices, and urgent care centers with highly accurate and rapid POC RT-PCR technology will be the way forward…
Podcast: One Practice’s Transformation Through In-Office PCR
From Pandemic to Tripledemic to Endemic: One Practice’s Transformation Through In-Office PCR.
Educational Materials
Select your area of interest
US-IVD. In Vitro Diagnostic Medical Device
Please see individual product inserts for full test run times and early assay termination times.
Please see Product inserts for individual test clearances.
*These tests have not been FDA cleared or approved. These tests have been authorized for emergency use by FDA under an EUA for use by authorized laboratories. Xpert Xpress CoV-2 plus has been authorized only for the detection of nucleic acids from SARS-CoV-2, and not for any other viruses or pathogens. Xpert Xpress CoV-2/Flu/RSV plus has been authorized only for the simultaneous qualitative detection and differentiation of nucleic acids from SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus (RSV), and not for any other viruses or pathogens. The emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.








