5 minuti di lettura
6 giugno 2025
TENDENZE A LIVELLO DI TECNOLOGIA E MALATTIE
Articolo
Innovation in Times of Need: Cepheid’s Legacy of Outbreak Response
A story of micro organisms and macro impact
Walk through a history of innovation in response to—and often ahead of—outbreak threats from emerging and evolving infectious agents. Read about the Cepheid innovations that have been deployed to address infectious public health emergencies for over 20 years, and the foundations laid to prepare for future needs.
The first Cepheid cartridge for crisis response: Anthrax
In September 2001 the world was rocked by devastating terrorist attacks forever memorialized as ‘9/11’. Just one month later, another series of attacks besieged the U.S. when four letters laced with anthrax spores were sent through the mail, resulting in 22 illnesses, 5 deaths, and widespread fear of repeat attacks.1
In response to the threat, the United States Postal Service (USPS) commissioned the first-ever rapid test for biohazards in the mail system. Cepheid partnered with Northrop Grumman, a multinational aerospace and defense company, to develop its first cartridge-based test to detect anthrax spores in the early 2000s. The USPS still uses the test today as part of its Biohazard Detection System2 installed at all postal plants nationwide to help safeguard its employees and customers from anthrax.

Cepheid Anthrax Cartridge on post office desk
Response to the “Swine Flu” Pandemic: the First Xpert® Flu Test
By the mid-2000s, Cepheid had begun providing molecular tests to hospitals and clinics in an automated, cartridge-based format, simplifying clinical molecular diagnostics for pathogens like Group B Streptococcus and methicillin-resistant Staphylococcus aureus.
In 2009, a novel influenza A (H1N1) virus initially dubbed “Swine Flu” emerged in North America and quickly spread around the world, resulting in the first influenza pandemic of the 21st century.3 This new virus contained a unique combination of influenza genes, previously unknown in animals or humans, creating a dire need for diagnostic tests to identify the virus in patient samples.
Following the declaration of a public health emergency due to the H1N1 outbreak, the U.S. Food & Drug Administration (FDA) authorized the emergency use of selected in vitro diagnostic tests.4 Cepheid responded to this call with rapid innovation and obtained Emergency Use Authorization (EUA)4 for the Xpert Flu test in 2009. Although the WHO declared an end to the 2009 H1N1 influenza pandemic in August 2010, the H1N1 virus continues to circulate as a seasonal flu virus, causing illness, hospitalization, and deaths each year,5 making the test’s targets relevant still today.
With a novel multiplexed test design, Xpert Flu included detection targets for Flu A, Flu B, and a dedicated H1N1 reportable result. Cepheid’s first EUA was significant and laid the foundation for another outbreak response 10 years later.

Alert piglet innocent to its transmission role
Multiplex Panel Testing and the Agility to Deal with Variants: Flu + RSV
Influenza viruses constantly change, creating new variants in their unending game of molecular hide-and-seek. Cepheid responded to emerging avian flu variants by further updating its Xpert Flu test in 2012 to increase Flu A strain coverage to detect H5N1.
To stay ahead of viral mutation and avoid constantly updating its tests, Cepheid turned to the power of multi-target test design to create a powerhouse viral respiratory test. In 2014, multiplexing innovations were implemented to develop one of the first Flu+RSV combination molecular tests on the market, Xpert Flu/RSV XC (extended coverage).
This was designed to be a “pandemic-ready” flu test with broader Flu A coverage, detection of the emerging H3N2 variant, more reliable Flu B detection, and the addition of RSV A and B in a combined RSV call-out. The test was launched in both U.S. (FDA) and European (CE-IVD) markets in 2014. In 2015, the test obtained U.S. FDA CLIA waived status, becoming the first CLIA waived PCR panel test, available for use in point-of-care settings.

Hand holding H1N1 sample vial in front of chicken
Responses to Ongoing Global Health Crises: Tubercolosi
Tuberculosis (TB) continues to be a global epidemic and is the leading cause of infectious disease mortality worldwide, with over 1:25M annual deaths.6 Cepheid has supported TB testing in high-burden countries and worldwide for over 15 years. Xpert MTB/RIF, released in 2009 in collaboration with the Foundation for Innovative New Diagnostics (FIND), was a first-of-its-kind molecular test that could simultaneously detect Mycobacterium tuberculosis (the bacterium that causes TB) and potential resistance to rifampin, one of the most powerful TB drugs.
The test was endorsed by the World Health Organization (WHO) in 2010. In a global press conference, Marion Raviglione, Director of Stop TB commented, “It’s basically a development that the world has been waiting for, for literally decades.”7 According to Reuters,8 WHO said in a statement it was endorsing the test because it could "revolutionize" TB care and control by accurately diagnosing patients in about 100 minutes, compared to current tests that can take up to three months to provide results.
Further innovations in the fight against TB included the Xpert MTB/RIF Ultra test with an updated design and faster results and Xpert MTB/XDR in collaboration with FIND to address the increasing problem of extensively drug-resistant (XDR)9 TB, the most serious form of TB. Xpert MTB/XDR leveraged increased multiplexing capability that enables the detection of multiple resistance-conferring mutations across several genes, from a single sample.
Innovations are not limited to test design, however. In 2011, Cepheid launched its Global Access program in partnership with key non-profit organizations, to provide special pricing for high-burden developing countries. Today, the program reaches every corner of the globe impacted by deadly diseases like TB, HIV, and Ebola.

Doctor holding GeneXpert testing cartridge
Aiming to Stop Deadly Diseases in their Tracks: Ebola
2024 marked the 10-year remembrance of the largest Ebola epidemic in history.10 Early testing was crucial because, without treatment, Ebola’s fatality rate is up to 90%.10 With the number of infections roughly doubling every 3-4 weeks and without a safe and effective vaccine, identifying infected patients and quarantining them was essential for controlling the spread of disease.11 Yet, fewer than one in five people with Ebola was diagnosed within two days of becoming infectious, even nearly a year into the 2014 epidemic.11
The Xpert Ebola test obtained EUA and WHO eligibility listing for procurement to Ebola-affected countries in 2015 and was deployed to West Africa and other countries in need. Two years later, when a new Ebola outbreak began in the Democratic Republic of the Congo, Cepheid’s test was recognized for its role in containing the spread.12 The DRC’s government made GeneXpert system its primary method of testing for Ebola during the outbreak, allowing for fast results and a more nimble response than was possible during the earlier epidemic.12
Provider draws blood from patient for sample testing
Withstanding Genetic Variability with Broad Strain Coverage: The COVID-19 Pandemic
When the COVID-19 pandemic was declared in 2020, Cepheid had the test design expertise to respond in record time. Xpert Xpress SARS CoV-2, released under U.S. FDA EUA in March 2020, was the first rapid SARS-CoV-2 PCR test for use at the point of care. Six months later, Cepheid’s 4-in-1 respiratory test received EUA to detect SARS-CoV-2, Flu A, Flu B, and RSV: co-circulating viruses that have overlapping clinical presentations but different clinical management.
Given the rapid evolution of the coronavirus and the expectation that new viral variants would arise over time, Cepheid updated the tests in 2021 by adding a third gene target for SARS-CoV-2 detection. With three genes available to detect the novel coronavirus, Cepheid’s tests withstood the genetic variability that impacted other tests,13 enabling healthcare providers and patients to benefit from reliable SARS-CoV-2 results during the pandemic.
Provider obtains nasal swab sample from patient for testing
Controlling a Recent Outbreak with Preparation: Mpox (vaiolo delle scimmie)
In May 2022, an outbreak of mpox emerged and quickly spread across Europe, the Americas, and all six WHO regions. It was soon declared a public health emergency of international concern,14 a formal declaration by the WHO of an extraordinary event that poses a public health risk to other countries through the international spread of disease and potentially requires a coordinated international response.
Xpert Mpox was the first point-of-care test to receive EUA from the U.S. FDA for the detection of mpox infection in 2023. As the only point-of-care molecular test with WHO Emergency Use Listing (EUL), it was a valuable preparedness and response tool that could be deployed quickly where accurate and actionable information was needed. Xpert mpox proved valuable again in 2024 when a new international mpox emergency was declared, requiring quick and accurate diagnosis to support affected communities.
Looking to the Future
Molecular diagnostics must be agile and accessible to address public health challenges and disease outbreaks. Future developments must focus on enhancing rapid testing capabilities, improving multiplexing technologies, and ensuring adaptability to emerging pathogens and clinical requirements.
Whatever our next call to action may be, Cepheid is prepared to meet the needs of healthcare providers, patients, and communities worldwide. We are ready to serve and continuously innovating for what’s ahead.
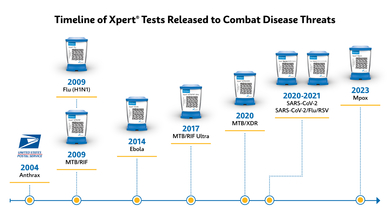
Timeline of Xpert tests released to combat disease threats
Xpert tests are IVD. In vitro diagnostic medical devices.
Not all tests mentioned are commercially available. Tests may not be registered in all regions.
Xpert Flu, Xpert Xpress CoV2 plus, and Xpert Xpress CoV/Flu/RSV plus were originally available under U.S. FDA Emergency Use Authorization only. These tests were subsequently cleared/marked as U.S. and CE-IVD.
Xpert Ebola is available under U.S. FDA Emergency Use Authorization only.
Xpert MTB/RIF Ultra and Xpert MTB/XDR are CE-IVD, not available in the U.S.
Riferimenti bibliografici:
1. Storia | Federal Select Agent Program. Accesso effettuato il 17 marzo 2025. https://www.selectagents.gov/overview/history.htm#:~:text=In%20October%202001%2C%20bioterrorism%20in,exposed%20totaled%20over%20$23%20million.
2. https://about.usps.com/news/state-releases/sc/2013/FAQ-BDS.pdf. Consultato il 17 marzo 2025. https://about.usps.com/news/state-releases/sc/2013/FAQ-BDS.pdf
3. Al Hajjar S, McIntosh K. The first influenza pandemic of the 21st century. Ann Saudi Med. 2010;30(1):1-10. doi:10:4103/0256-4947:59365
4. U.S. FDA. Federal Register: Authorization of Emergency Use of Certain In Vitro Diagnostic Devices; Availability. Accessed 17 marzo 2025. https://www.federalregister.gov/documents/2010/4/2019/2010-8605/authorization-of-emergency-use-of-certain-in-vitro-diagnostic-devices-availability
5. CDC. 2009 H1N1 Pandemic (H1N1pdm09 virus) | Pandemic Influenza (Flu) | CDC. Consultato il 17 marzo 2025. https://archive.cdc.gov/www_cdc_gov/flu/pandemic-resources/2009-h1n1-pandemic.html
6. Tuberculosis resurges as top infectious disease killer. Consultato il 18 marzo 2025. https://www.who.int/news/item/29-10-2024-tuberculosis-resurges-as-top-infectious-disease-killer
7. WHO Endorses New Rapid Diagnostic Test for TB | Working Group for New TB Drugs. Consultato il 17 marzo 2025. https://www.newtbdrugs.org/news/who-endorses-new-rapid-diagnostic-test-tb
8. Reuters.com. Consultato il 17 marzo 2025. https://www.reuters.com/article/us-tuberculosis-test/who-says-cepheid-rapid-test-will-transform-tb-care-idUSTRE6B71RF20101208/
9. WHO announces updated definitions of extensively drug-resistant tuberculosis. Accesso effettuato il 17 marzo 2025. https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis#:~:text=Extensively%20drug%20resistant%20TB%20is%20a%20more,cornerstone%20medicines%20for%20the%20treatment%20of%20TB.
10. Outbreak History | Ebola | CDC. Accesso effettuato il 17 marzo 2025. https://www.cdc.gov/ebola/outbreaks/index.html#:~:text=The%20Ebola%20virus%20is%20the,more%20than%2028%2C600%20cases%20reported.
11. Ebola experts seek to expand testing | Nature. Consultato il 17 marzo 2025. https://www.nature.com/articles/516154a
12. Butler D. Speedy Ebola tests help contain Africa’s latest outbreak. Nature. 2018;558(7709):172. doi:10:1038/d41586-018-05389-2
13. Bano I, Sharif M, Alam S. Genetic drift in the genome of SARS COV-2 and its global health concern. J Med Virol. 2022;94(1):88-98. doi:10:1002/jmv.27337
14. Mpox. Consultato il 17 marzo 2025. https://www.who.int/news-room/fact-sheets/detail/mpox
Leggi successivo
Altro