
Resistance Plus ® MG FleXible
M. genitalium+ macrolide resistance detection
Sign in or create a MyCepheid account to add items to cart
Test pack size(s)

10 Tests
S2A-95004
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.

10 Tests
S2A-2000410
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Collection devices

ASWAB Dual Swab Specimen Collection Kit (Pack of 50)
SWAB/A-50
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.

Swab Specimen Collection Kit (Pack of 50)
SWAB/G-50
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.

Urine Collection Kit (Pack of 50)
URINE/A-50
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Total
{{currency}}
0
Error adding items to cart. If this error persists, please contact Digital Support
The Need
M. genitalium is a recognised Sexually Transmitted Infection (STI), treated syndromically, with clinical presentation similar to that of Chlamydia trachomatis.1
Prevalence of M. genitalium infections in the general population ranges from 1-4%2, and its treatment is challenging due to high levels of macrolide resistance.5
Prevalence of M. genitalium infections in the general population ranges from 1-4%2, and its treatment is challenging due to high levels of macrolide resistance.5
- Macrolide resistance testing is recommended by international and local guidelines on management of M. genitalium infections.3,4,6-8
- Macrolide resistance testing can guide appropriate treatment choice enabling Resistance Guided Therapy.6
- Resistance Guided Therapy is clinically demonstrated to improve patient cure rate and overall patient management.9
- Fast detection of macrolide resistance can reduce time to cure, preventing ongoing transmission.9
1.Manhart LE and Kay N.Mycoplasma genitalium: Is It a Sexually Transmitted Pathogen? Curr. Infect. Dis. Reps. 2010; 12(4):306-313.
2. Cools et al, Lancet Infectious DiseasesVol. 20No. 11p1222–1223
3. 2018 BASHH UK national guideline for the management of infection withMycoplasma genitalium. Available online at https://www.bashhguidelines.org/media/1198/mg-2018.pdf
4. Jensen et al. 2016 European guideline onMycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016 Oct;30(10):1650-1656.
5. Unemo, M et al. Antimicrobial-resistant sexually transmitted infections: gonorrhoea andMycoplasma genitalium.Nat Rev Urol. 2017 Mar;14(3):139-152.
6. Horner PJ et al. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016 Oct;27(11):928-37.
7. Australian STI Management Guidelines – Mycoplasma genitalium 2018. http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium
8. Groupe Infectiologie Dermatologique et Infections Sexuellement GRIDIST and Société Fançaise de Dermatologie – Press Release. Available online at: https://www.sfdermato.org/actualites/communique-commun-gridist-et-sfd.html
9. Read TRH et al. Outcomes of Resistance-guided Sequential Treatment of Mycoplasma genitalium Infections: A Prospective Evaluation. Clin Infect Dis. 2019 Feb 1;68(4):554-560.
2. Cools et al, Lancet Infectious DiseasesVol. 20No. 11p1222–1223
3. 2018 BASHH UK national guideline for the management of infection withMycoplasma genitalium. Available online at https://www.bashhguidelines.org/media/1198/mg-2018.pdf
4. Jensen et al. 2016 European guideline onMycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016 Oct;30(10):1650-1656.
5. Unemo, M et al. Antimicrobial-resistant sexually transmitted infections: gonorrhoea andMycoplasma genitalium.Nat Rev Urol. 2017 Mar;14(3):139-152.
6. Horner PJ et al. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016 Oct;27(11):928-37.
7. Australian STI Management Guidelines – Mycoplasma genitalium 2018. http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium
8. Groupe Infectiologie Dermatologique et Infections Sexuellement GRIDIST and Société Fançaise de Dermatologie – Press Release. Available online at: https://www.sfdermato.org/actualites/communique-commun-gridist-et-sfd.html
9. Read TRH et al. Outcomes of Resistance-guided Sequential Treatment of Mycoplasma genitalium Infections: A Prospective Evaluation. Clin Infect Dis. 2019 Feb 1;68(4):554-560.
The Solution
- ResistancePlus® MG FleXible detects both M. genitalium and macrolide resistance in ~ 2 hours.
- ResistancePlus® MG FleXible offers a fast and simple solution in combination with GeneXpert®Systems. Easily implemented into your existing workflow or setting, with minimal preparation time ~ 10 mins.
- High performance test validates a wide range of specimen types and collection devices, including urine and swabs (vaginal, cervical, rectal).**
**Validated specimens: male and female urine, vaginal swab, cervical swab, rectal swab, urethral swab, from symptomatic and asymptomatic patients. Validated collectionkits: Xpert® Vaginal/Endocervical and Xpert® Urine Specimen CollectionKits, Neat urine, Regular FLOQSwab™ in 3 ml of UTM™ media, cobas PCR collection media. For details refer to theResistancePlus®MG FleXible Instructions For Use (IF-IV0012)
SWAB-G-50
SWAB-G-50
The Impact
Improving patient management
ResistancePlus®MG FleXible provides therapeutic guidance recommendations, enabling clinicians to make informed treatment decisions. Resistance guided therapy is clinically demonstrated to increase overall patient cure rate.9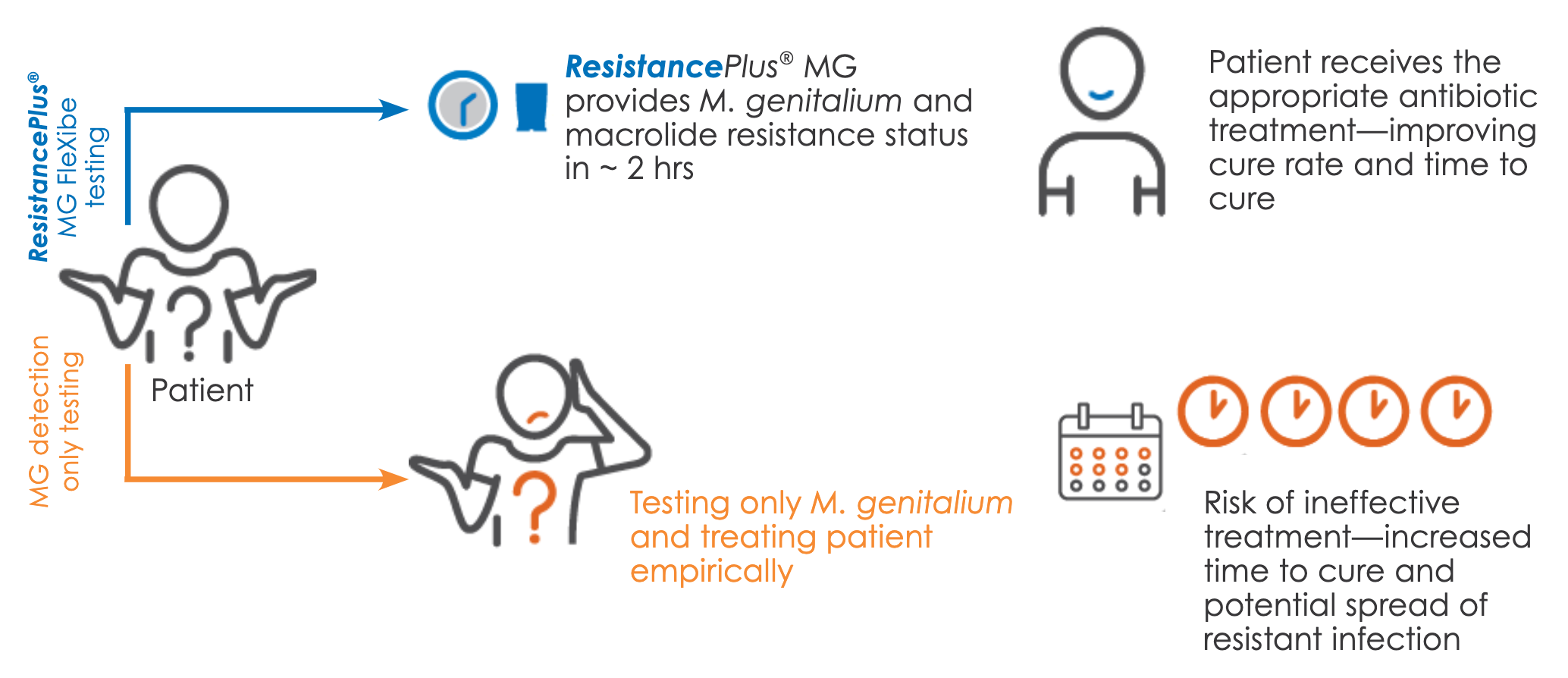
ResistancePlus®MG FleXible is validated on a wide range of sample types including rectal, male and female urine, and common collection swab kits including Xpert®CT/NGVaginal/Endocervical Specimen Collection kit and Xpert®CT/NG Urine Specimen Collection Kit.**
CE-IVDin VitroDiagnostic Medical Device. Not available in all countries.
*Exclusively distributed by Cepheid under the FleXible by GeneXpert® System program
ResistancePlus® MG FleXible tests are developed and manufactured by SpeeDx Pty Ltd, Sydney. PlexPCR® &ResistancePlus® are trademarks of SpeeDx Pty Ltd. Other copyright and trademarks are theproperty of the respective owners. SpeeDx Pty Ltd productsmaybe covered by oneormore localor foreign patents.Visitwww.plexpcr.com/patentsfor comprehensivepatentinformation
*Exclusively distributed by Cepheid under the FleXible by GeneXpert® System program
ResistancePlus® MG FleXible tests are developed and manufactured by SpeeDx Pty Ltd, Sydney. PlexPCR® &ResistancePlus® are trademarks of SpeeDx Pty Ltd. Other copyright and trademarks are theproperty of the respective owners. SpeeDx Pty Ltd productsmaybe covered by oneormore localor foreign patents.Visitwww.plexpcr.com/patentsfor comprehensivepatentinformation






