
Xpert® SA Nasal Complete
Pre-surgical S. aureus and MRSA in about 65 minutes
Sign in or create a MyCepheid account to add items to cart
Test pack size(s)

10 Tests
GXSACOMP-CE-10
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Collection devices
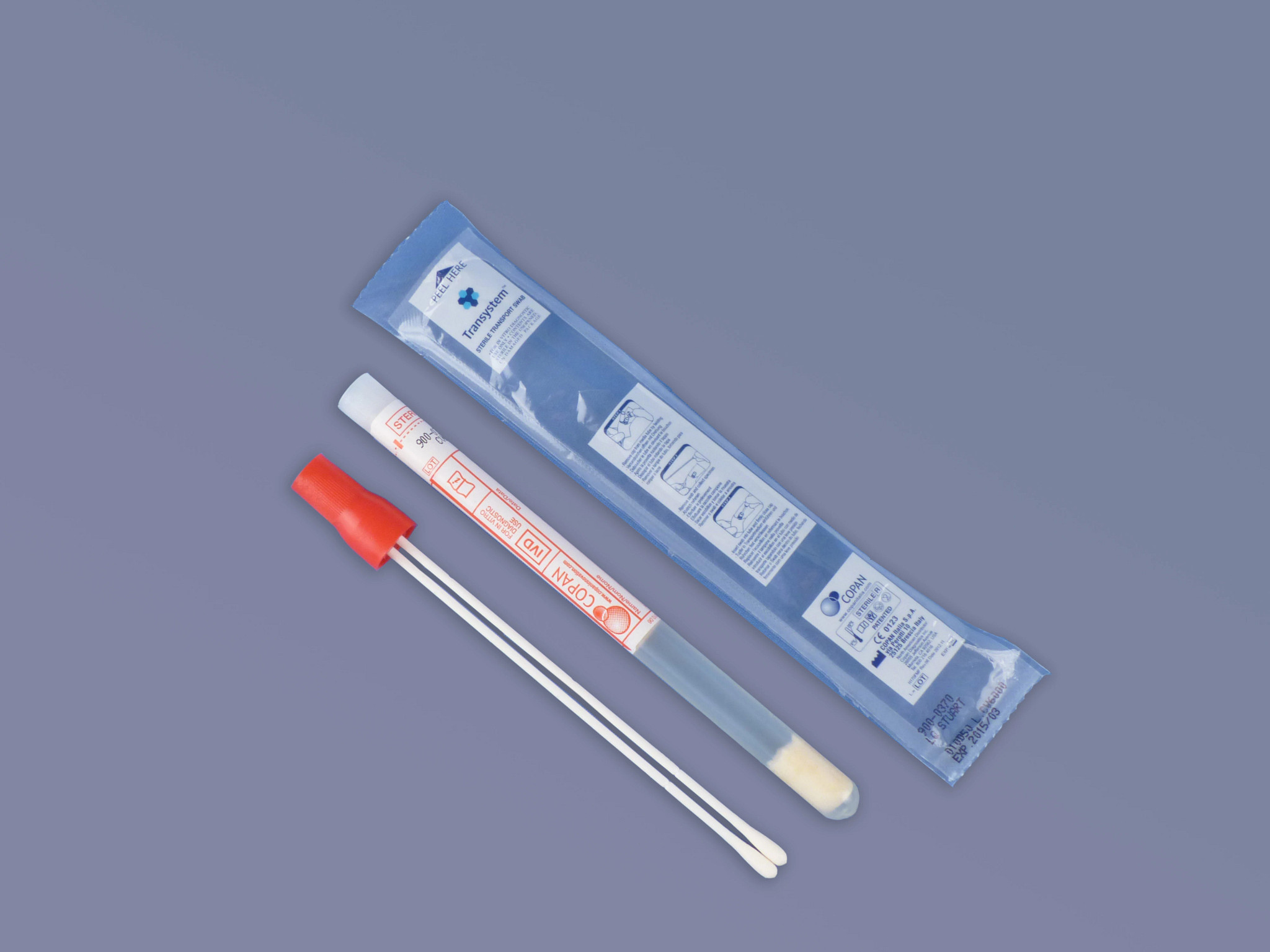
Collection Device (Pack of 50)
900-0370
Qty
Unit price
Subtotal
USD
Product is not available for purchase in your region.
Total
{{currency}}
0
Error adding items to cart. If this error persists, please contact Digital Support
The Need
S. aureus: An Ongoing Threat
- Patients undergoing surgery are at increased risk of developing an infection1,2
- S. aureus is the most common cause of SSIs1
- Colonized patients are up to nine times more likely to develop an SSI1
- SSIs are associated with increases in length of stay, cost, morbidity, and mortality1
- Traditional direct culture testing takes 24+ hours to produce a result, and may risk under-detecting positive cases2
(1) Kluytmans J, et al. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997 Jul;10(3):505-20.
(2) Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO J. 2000 Nov-Dec;46(6):S13-7.
(2) Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO J. 2000 Nov-Dec;46(6):S13-7.
The Solution
SSIs caused by S. aureus continue to present a substantive burden in healthcare settings, prompting the need for earlier and more accurate pre-admission identification.
Xpert® SA Nasal Complete enables:
Xpert® SA Nasal Complete enables:
- Fast identification and differentiation of S. aureus and MRSA in around 60 minutes
- Broad coverage with multiple targets, including: the orfXSCCmec junction, mecA gene and spa gene for more accurate S. aureus and MRSA detection
- Optimised pre-admissions pathway for improved patient outcomes
- Appropriate decolonization measures to reduce SSIs and the development of new resistances
- Laboratory efficiencies with on-demand workflows requiring minimal hands-on time
The Impact
Fast and accurate PCR testing with Xpert® SA Nasal Complete greatly improves time to result, allowing healthcare professionals to quickly and appropriately manage pre-surgical admissions, reducing infection risk, transmission, and patient length of stay.
Optimize Workflow and Patient Management
Optimize Workflow and Patient Management
- Deliver fast and actionable results to surgical and infection control teams in around 60 minutes
- On-demand STAT testing provides results 24/7
- Ease of use and minimal hands-on-time enables more efficient use of staff time
(3) Critchley IA. Eradication of MRSA nasal colonization as a strategy for infection prevention. Drug Discov. Today. 2006;3(2):189-95.
(4) Yu VL, et al. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91-6.
(5) Murphy D, et al. Dispelling the Myths: The True Cost of Healthcare-Associated Infections. An APIC Briefing. 2007 Feb. Accessed May 2020. http://www.thainapci.org/download-guideline-6.pdf/
(6) Engemann JJ, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003 Mar 1;36(5):592-8. Epub 2003 Feb 7.
(7) Anderson, DJ. Clinical and Financial Outcomes Due to Methicillin Resistant Staphylococcus aureus Surgical Site Infection: A Multi-Center Matched Outcomes Study. PLOS One. 2009 Dec 15. Accessed May 2020. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0008305
(8) Bode LG, et al. Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus aureus. N Engl J Med. 2010 Jan 7;362:9-17.
(9) Mackie DP, et al. Reduction in Staphylococcus aureus wound colonization using nasal mupirocin and selective decontamination of the digestive tract in extensive burns. Burns. 1994;20 Suppl 1:S14-7; discussion S17-8.
(10) Kooistra-Smid M, et al. Molecular epidemiology of Staphylococcus aureus colonization in a burn center. Burns. 2004 Feb;30(1):27-33.
(11) Noskin GA, et al. The Burden of Staphylococcus aureus Infections on Hospitals in the United States. Arch Intern Med.2005;165(15):1756–1761.
(4) Yu VL, et al. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91-6.
(5) Murphy D, et al. Dispelling the Myths: The True Cost of Healthcare-Associated Infections. An APIC Briefing. 2007 Feb. Accessed May 2020. http://www.thainapci.org/download-guideline-6.pdf/
(6) Engemann JJ, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003 Mar 1;36(5):592-8. Epub 2003 Feb 7.
(7) Anderson, DJ. Clinical and Financial Outcomes Due to Methicillin Resistant Staphylococcus aureus Surgical Site Infection: A Multi-Center Matched Outcomes Study. PLOS One. 2009 Dec 15. Accessed May 2020. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0008305
(8) Bode LG, et al. Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus aureus. N Engl J Med. 2010 Jan 7;362:9-17.
(9) Mackie DP, et al. Reduction in Staphylococcus aureus wound colonization using nasal mupirocin and selective decontamination of the digestive tract in extensive burns. Burns. 1994;20 Suppl 1:S14-7; discussion S17-8.
(10) Kooistra-Smid M, et al. Molecular epidemiology of Staphylococcus aureus colonization in a burn center. Burns. 2004 Feb;30(1):27-33.
(11) Noskin GA, et al. The Burden of Staphylococcus aureus Infections on Hospitals in the United States. Arch Intern Med.2005;165(15):1756–1761.










