Les patients sont notre priorité.
Une prise en charge efficace des patients commence par des résultats de tests plus rapides.
Cepheid s’engage à améliorer les résultats des patients dans le monde entier grâce à des tests rapides et précis pour les maladies telles que la tuberculose, le VHC et les maladies respiratoires.
Sélectionnez votre domaine d’expertise
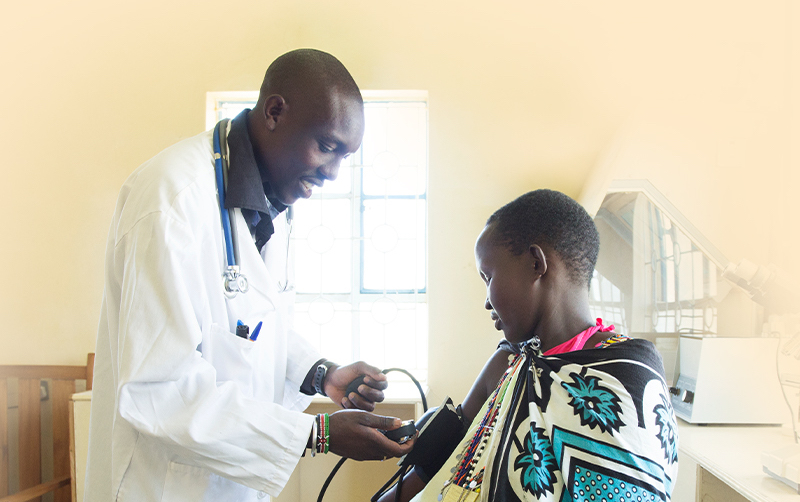
Santé publique mondiale
Le Programme d’accès mondial (Global Access Program) de Cepheid repose sur la conviction que tout le monde, partout, devrait avoir accès à des tests de diagnostic de haute qualité.
De meilleures réponses conduisent à de meilleurs résultats.
Un système. De multiples tests.
Le système GeneXpert® de Cepheid est une plateforme flexible et entièrement évolutive qui fournit aux professionnels de santé une large gamme de tests, quel que soit le contexte, du laboratoire central jusqu’aux applications proches du patient. Découvrez pourquoi les solutions de Cepheid constituent le meilleur choix pour des tests diagnostiques rapides, précis et simples.

Nos formations, ressources et outils peuvent vous ouvrir la voie vers une réussite constante, où que
vous alliez.
